About CMUH
Proposed AI Implementation Process
:::
About CMUH
Proposed AI Implementation Process
Principles
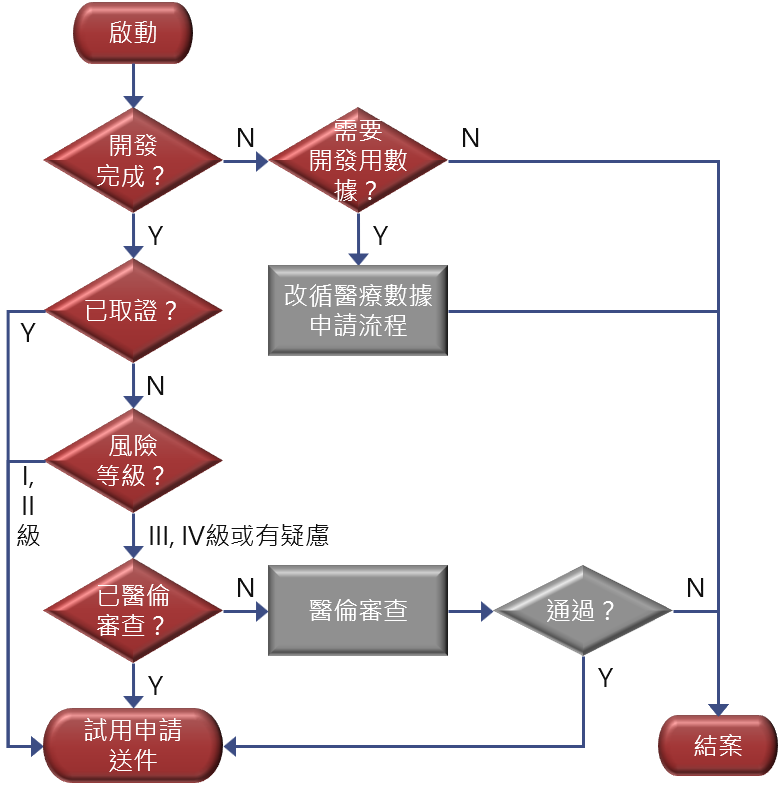
- Applies to fully developed medical-AI systems intended to interface with CMUH HIS and collect clinical data for performance evaluation.
- Medical-AI developed in-house or holding a Taiwan FDA (TFDA) marketing authorization is given priority for trial.
- Medical-AI without a license must first obtain CMUH Institutional Review Board (IRB) approval before applying for trial and subsequent production.
- High‑risk medical-AI (Class III or IV) must obtain IRB approval and complete clinical trials before it may apply for trial and production.
* Adapted from U.S. FDA risk‑stratification guidance.Risk Class Use Context for Inference Output Treatment / Diagnosis Drives Clinical Management Informs Clinical Management Clinical Condition Critical IV III II Serious III II I Non‑serious II I I Treatment / Diagnosis: Direct basis for a physician’s treatment or diagnostic decision.
Drives clinical management: Triggers secondary assessment or intervention by healthcare staff.
Informs clinical management: Reference only; final diagnosis/decision remains with healthcare staff. - Applicants must:
- Provide metrics and reference standards for the safety and effectiveness of the medical-AI;
- Cooperate in continuous monitoring and periodic reporting to enable life‑cycle management;
- Submit the Nine‑Point Transparency Disclosure and explainability analysis, publicly accessible to all stakeholders.
- The inference server may be provided by CMUH or by the applicant. When using a CMUH server, installation is the applicant’s responsibility.
- The medical‑AI system should be loosely coupled with existing hospital IT systems and must not disrupt their operation.
- CMUH has issued the AI‑on‑HIS FHIR Implementation Guide for integration.
- Alternative integration methods must first be reviewed for technical feasibility by the IT Office, and any additional costs borne by the applicant.
- The medical‑AI system must comply with the Cybersecurity Management Act, the Personal Data Protection Act, and relevant CMUH regulations and ISO frameworks.
- By default, medical‑AI must not connect to external networks.
- If external connectivity is required, the applicant must submit a detailed security plan and bear associated costs.
- Unauthorized external connections are strictly prohibited and will be reported to the Personnel Evaluation Committee.
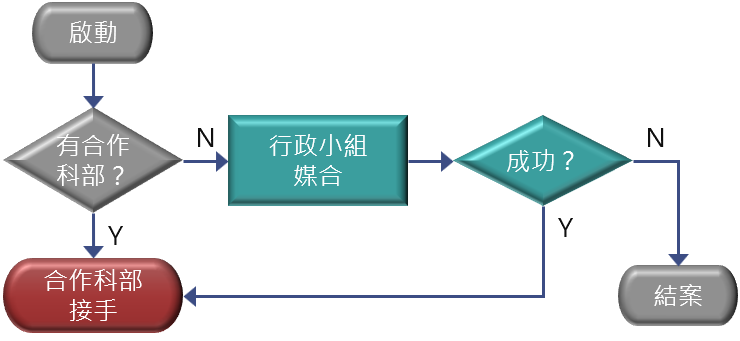
- Trial and production applications should be submitted by CMUH employees. External organizations should apply via a CMUH partner department or contact the Center for assistance.
Internal Department Workflow
External Organization Workflow
Trial Application
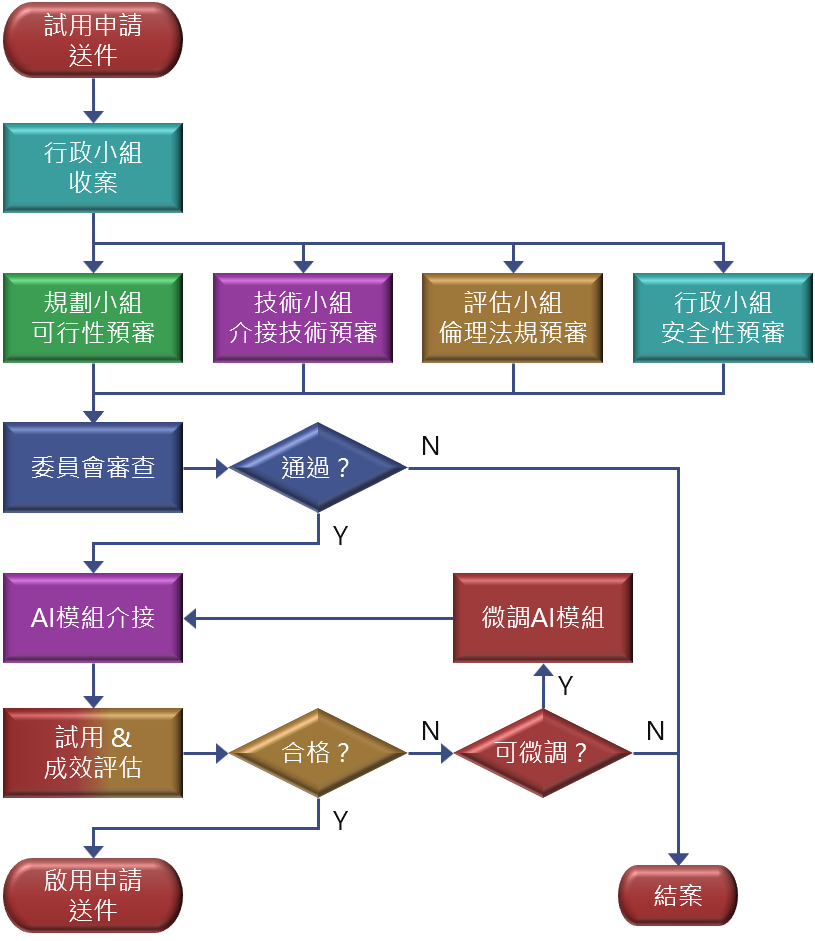
A trial is a small‑scale evaluation of medical‑AI in the HIS. Clinical decisions remain solely at the discretion of healthcare professionals.
Steps
- Download and complete the Medical‑AI Software Trial Application Form.
- Fill out the Information‑System Security Baseline Self‑Assessment according to the required security level.
- We recommend pre‑filling the Nine‑Point Transparency Disclosure Form.
- Submit an “AI2 Medical‑AI Software Go‑Live Request” in the BPM e‑sign system and select Trial.
- The Smart Healthcare Committee reviews:
- Feasibility and necessity;
- Integration technical suitability;
- Ethics and regulatory compliance;
- Safety, including cybersecurity and personal‑data protection.
- Only after approval may the AI connect to HIS and start the trial.
- System go‑live follows existing ISO procedures of the IT Office.
- At trial end, evaluate performance per the plan in the application.
Flowchart
Production Application
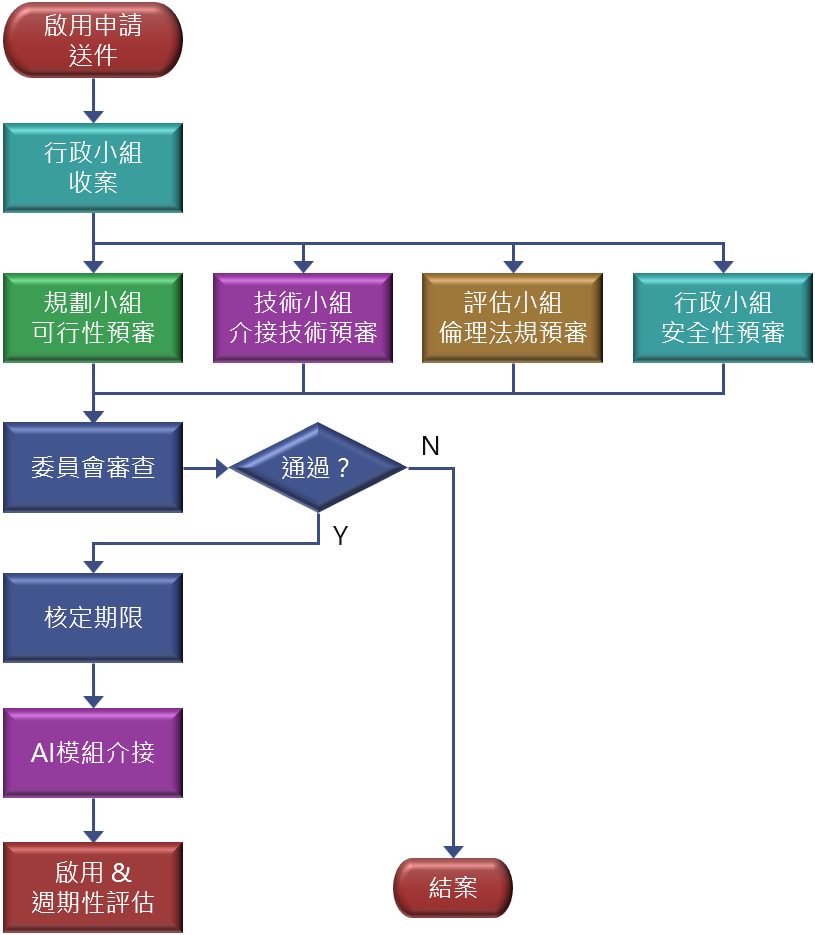
Production refers to a trial‑approved medical‑AI whose performance meets requirements and is fully integrated into clinical workflows.
Steps
- Download and complete the Medical‑AI Software Production Application Form.
- Attach trial performance‑evaluation results.
- Fill out the Information‑System Security Baseline Self‑Assessment.
- Submit the mandatory Nine‑Point Transparency Disclosure Form.
- Submit an “AI2 Medical‑AI Software Go‑Live Request” in BPM and select Production.
- The Committee reviews and grants a production period.
- Only after approval may the AI formally connect to HIS.
- Go‑live follows standard ISO procedures.
Flowchart
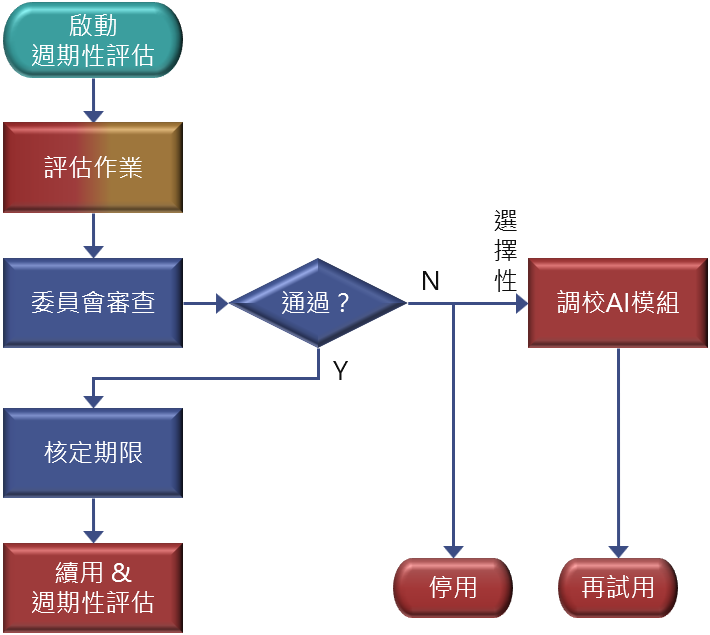
Life‑cycle Monitoring & Management
- Evaluate performance periodically as defined in the production application.
- Before the production period expires, submit evaluation results to apply for extension.
- The Committee conducts periodic reviews and decides on continuation or retirement.
- Retired AI may reapply for trial after being retrained or adjusted.
Flowchart
AI2 Medical‑AI Software Go‑Live Request (Form Screenshot)
.png)
▲
